At what temperature does water freeze? Under normal atmospheric pressure, water freezes at 32 degrees Fahrenheit (0°C or 273.15 Kelvin). That is the freezing temperature of water taught in classrooms worldwide. However, water does not always turn to ice at exactly 32°F. In certain controlled conditions, liquid water can remain unfrozen far below this point—sometimes as low as -40°F or even -42°F. Understanding why requires looking beyond simple textbook definitions.
The question “what temperature does water freeze?” appears straightforward. Yet the physics of freezing reveals a more nuanced reality shaped by molecular structure and environmental conditions.
What Temperature Is Freezing in Fahrenheit Under Normal Conditions?
In everyday settings, when does water freeze in Fahrenheit? The practical answer remains 32°F. This is the point at which liquid water and solid ice coexist in equilibrium under standard pressure.
So if you ask what temp does water freeze at during a winter night or inside your freezer, the answer is typically 32°F or lower. The phrase water freezes at what temperature is most accurately answered with 32°F under ordinary atmospheric conditions.
For clarity:
Table: Standard Freezing Points of Water
Scale | Freezing Temperature of Water
Fahrenheit | 32°F
Celsius | 0°C
Kelvin | 273.15 K
These values apply when pressure is normal and impurities are absent.
Why Water Does Not Always Freeze at 32°F
The common assumption that water freezes at 32°F overlooks a key scientific factor: nucleation. For ice crystals to form, water molecules require a seed or nucleus around which crystallization begins.
In everyday environments, dust, minerals or container surfaces provide these nucleation points. But in very pure water, lacking such impurities, freezing behaves differently.
This explains why asking what temp.does water freeze sometimes yields surprising laboratory results.
Supercooling: When Water Stays Liquid Below 32°F
Scientists have documented liquid water at temperatures as low as -40°F in clouds. In laboratory conditions, water has been cooled to -42°F without freezing.

This phenomenon is called supercooling. It occurs because water molecules, although cold enough to freeze, do not immediately organize into the structured lattice required for ice formation.
Supercooling shows that the answer to what temp does water freeze is not fixed in every circumstance.
The key question becomes: how far can supercooling extend?
Molecular Structure and the Role of Tetrahedrons
Research by Emily Moore and Valeria Molinero at the University of Utah used advanced computer simulations to examine this behavior. Their model simulated 32,768 water molecules to analyze changes in heat capacity, density and compressibility during cooling.
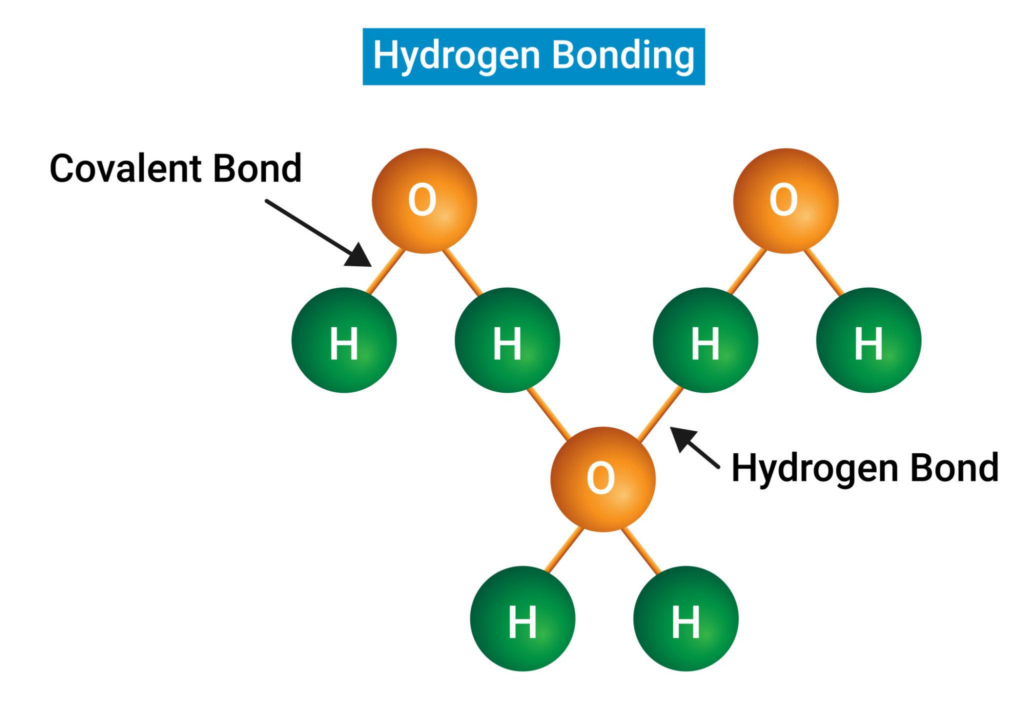
As water approaches approximately -55°F, molecular structure changes significantly. Molecules begin forming tetrahedral arrangements, loosely bonding with four neighboring molecules.
This structural shift mirrors the geometry of solid ice. As this transformation occurs:
- Density decreases
- Heat capacity increases
- Compressibility rises
Once water molecules adopt this ice-like structure, crystallization accelerates.
Below roughly -55°F, liquid water can exist only momentarily before freezing occurs almost instantly.
What Temp Water Freeze: The Structural Threshold
So at what temperature does water freeze in extreme cases? Experimental data suggest that around -55°F marks a structural limit for sustained liquid behavior.
However, below -42°F, freezing happens too rapidly for precise measurement of liquid temperature. This makes it difficult to determine an absolute lower boundary.
In practical terms:
- What temperature does water freeze in household conditions? 32°F.
- What temp does water freeze in ultra-pure laboratory environments? Possibly below -40°F.
- What temperature is freezing in Fahrenheit at structural instability? Near -55°F.
This distinction clarifies why textbooks and research papers can both be correct.
Why Nucleation Matters in Freezing
The presence of a nucleation site is decisive. Ice crystals require an organized starting point. In impure water, particles provide that anchor.
In pure water, spontaneous structural rearrangement must occur. That spontaneous reorganization is statistically unlikely until temperatures drop dramatically.
Molinero’s findings indicate that both thermodynamics and crystallization rate are controlled by structural shifts in liquid water.
Without a nucleus, even very cold water can remain liquid temporarily.
This explains why supercooled droplets exist in high-altitude clouds.
Real-World Implications of Supercooled Water
Understanding what temp does water freeze is not merely academic. Supercooled water droplets in clouds can freeze suddenly when disturbed, leading to icing on aircraft wings.

Meteorologists and aviation engineers must account for this phenomenon when modeling icing conditions.
Similarly, cryogenic research and climate modeling rely on accurate knowledge of the freezing temperature of water under varying pressures and purities.
In most everyday scenarios, the practical answer remains simple. But in technical contexts, nuance matters.
Pressure and Other Variables Affecting Freezing
The freezing temperature of water also shifts slightly with changes in pressure. At extremely high pressures, the freezing point can decrease.
However, under normal atmospheric conditions at sea level, 32°F remains the operational benchmark.
Thus, when does water freeze in Fahrenheit for everyday use? At 32°F. But in specialized scientific environments, additional variables intervene.
Why the Question Persists
The query at what temperature does water freeze persists because it bridges classroom simplicity and molecular complexity.
A student may answer 32°F correctly. A physicist may answer -42°F under supercooling conditions. Both answers are contextually valid.
The distinction lies in environmental purity, pressure and structural rearrangement.
In policy or infrastructure contexts—such as road safety, water supply systems or refrigeration design—the standard freezing temperature remains the reference.
In advanced research, the lower limits of supercooling remain an area of exploration.
A Simple Answer With Scientific Depth
So water freezes at what temperature? Under standard atmospheric pressure, water freezes at 32 degrees Fahrenheit. That remains the benchmark for practical purposes.

However, under controlled, impurity-free conditions, water can remain liquid far below 32°F due to supercooling. Structural transformation near -55°F appears to represent a practical lower threshold before rapid crystallization occurs.
The lesson is straightforward: context determines the answer. What temp does water freeze depends on purity, pressure and molecular behavior.
In everyday life, 32°F is sufficient. In laboratory science, the freezing point becomes a dynamic boundary shaped by molecular physics.
Understanding both perspectives ensures accuracy without oversimplification.